Roberto Saez Hernandez, Valencia University and Maria Luisa Cervera Sanz, Valencia University
O recent resurgence of a tuna food fraud strategy set off the alarms in the control institutions at European level. Hundreds of tuna pieces had to be analyzed as a result of suspicions that arose in the sector.
Some of the tuna samples were adulterated with unauthorized additives to change the final color of the piece. At this point, the reader may ask: why would the retailer be interested in tampering with the color of the tuna? How did they do it? In case of ingesting a fraudulent piece of tuna, do we run any risk as consumers?
In this article, we will try to answer these questions from a food chemistry perspective.
Why is tuna the color of tuna?
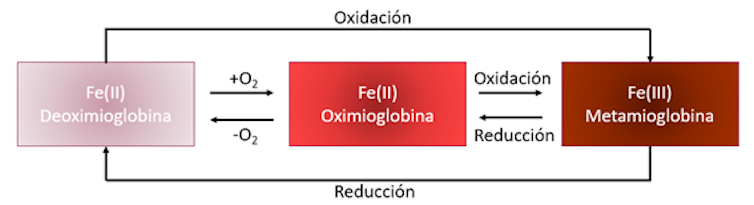
The characteristic color of the tuna is due to its hemoglobin and myoglobin content, complex organic molecules responsible for the transport of oxygen. In the case of gender thunnus, the content of myoglobin was shown to be higher than that of hemoglobin, so we will focus on your study.
In the structure of myoglobin there is an iron atom in the +2 –Fe (II) – oxidation state, responsible for binding to the O₂ molecule. The resulting structure is called oxymyoglobin and has a very bright red color that is attractive to the consumer.
Once the animal is captured and slaughtered, if it is deep-frozen at -40 ⁰C, its color remains with almost no apparent variation. However, this treatment is unusual and usually at freezing temperature post-capture is -18⁰C.
By thawing and filleting the tuna, the myoglobin is exposed to the air. This causes a color transformation from the initial deep red (due to oxyhemoglobin) to a reddish brown that is much less attractive to the client. This color change is linked to the oxidation of the iron atom, which goes from an oxidation state of +2 to +3. This new molecule is known as metmyoglobin.
It is evident that the longer the exposure to the environment, the greater the degree of appearance of metmyoglobin. Therefore, the worst aspect that the piece will present to buyers.
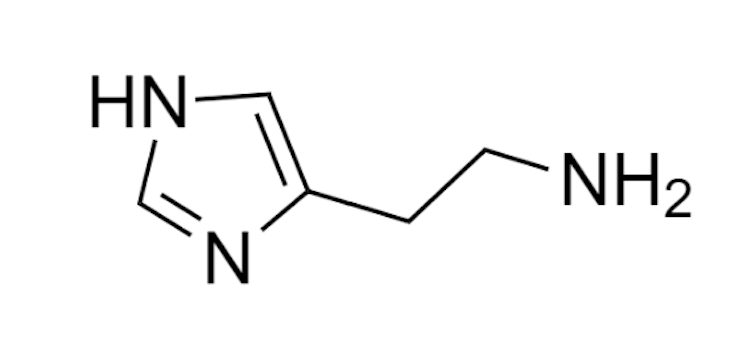
This is a good strategy for identifying the tuna catch date. A darker piece would be, a priori, less advisable due to the appearance of certain potentially toxic compounds, including biogenic amines. Of this group, perhaps the most significant is histamine, a nitrogenous compound originated from the decarboxylation of histidine, an amino acid that is very abundant in tuna.
Why adulterate the color of a tuna?
Tuna color adulteration has two reasons: on the one hand, the extension of its shelf life. On the other hand, make cheaper species pass for more expensive ones.
The first would seek to extend the time that the tuna could be on sale, as its appearance would remain palatable for longer.
The second consists of passing a piece of yellow fin tuna (thunnus albacares) or bigeye tuna (Thunnus obesus), lower price and quality species, for a bluefin tuna (Thunnus Thynnus) or bluefin tuna. The latter has its own coloration consisting of a characteristic very bright red that differentiates it from the rest of the cheaper species.
How is tuna color adulterated?
The main factor causing tuna color variation is the oxidation of myoglobin. Avoiding this step can prevent darkening. But how?
There are different strategies, including:
1. Addition of nitrate and nitrite salts (NO₃⁻ / NO₂⁻).
Its incorporation into the product provides a high concentration of these species in tuna meat. In this environment live a series of microorganisms capable of reducing nitrate to nitrite. This nitrite, in turn, is capable of generating highly reactive NO radicals, which can mimic the role that O₂ plays in myoglobin.
Thus, the color of oxymyoglobin would be artificially stabilizing itself.
2. Treatment of tuna with carbon monoxide.
Carbon monoxide, CO, plays the same role as the nitro compounds mentioned above. In the presence of CO, myoglobin would capture it, forming highly stable Mb-CO complexes with a very bright and striking red appearance.
3. Addition of plant extracts.
As the nitrate ion is very common in different plant species, its addition to fish such as tuna would generate an effect similar to the direct addition of nitrate salts.
Furthermore, thanks to the presence of pigments with a color very similar to that desired in beetroot, its extracts are particularly useful with regard to tuna adulteration.
Am I at risk if I eat adulterated tuna?
The above mentioned procedures were banned in the European Union for its ability to mislead consumers and for its potential health effects.
In the cases of tuna samples treated with carbon monoxide, this risk is lower, as the amount of this substance needed to have a visible effect is very small (<1% in CO already has a significant effect)
Conversely, if the adulteration was by addition of nitrate or nitrite salts, the risk may be greater. Nitrite has been shown to be able to generate reactions for the formation of nitrosamines, compounds related to the emergence of different types of cancer.
Furthermore, consumption of tuna outside the recommended period may involve certain dangers due to the appearance of the aforementioned biogenic amines. We previously highlighted histamine, as it is related to the onset of the syndrome called scombroidosis. It is characterized by feeling tingling, vomiting, dizziness and a burning sensation in the mouth after consuming a contaminated item.
conclusion
The emergence of a growing world market for tuna has created excessive demand that is difficult to cover. As a result, different strategies have been detected in order to be able to make available to the consumer foods that do not comply with European legislation.
Its accidental and sporadic consumption does not represent a great danger to the population. Furthermore, it is important to convey a reassuring message to the consumer: the fact that articles like this can describe the fraud detected demonstrates the effectiveness of official controls that offer us safe food.![]()
Roberto Saez Hernandez, Predoctoral Researcher FPU, Valencia University and Maria Luisa Cervera Sanz, Professor of Analytical Chemistry, Valencia University
This article was originally published in The conversation. read the original.